Cold Storage & How it Keeps Your Beer In Its Best Condition
“Okay everyone, chill!”
We’re pretty set in the middle of summer now, and we universally agree there’s absolutely nothing better than a cold beer that’s been sitting in the fridge at the end of a long, hot day to take the edge off the heat. How you store your beer prior to this glorious first sip is of just as much importance as enjoying it at the right temperature though – with this in mind, here’s a little stream of consciousness deep dive into the importance of cold storage and how it remains vital to a beer once it’s left the brewery gates.
It’s important to remember first and foremost that beer itself is a live product. In the same way a pint container of milk will have adverse reactions when left out in the open, the same can be said for beer, particularly craft beer. We do not pasteurise our beers here at Polly’s; we feel the pasteurisation process strips out a huge amount of aromatics, flavour compounds, and mouthfeel that we feel are the key components that make a Polly’s beer a Polly’s beer, and a cheap macro-produced imitation exactly that.
We’re extremely proud of the fact that every beer produced here at Polly’s is stored at four degrees for the entirety of its stay here at the brewery before it heads off to customers far and wide. We started back in 2018 with a single converted refrigeration trailer, and now have six all working simultaneously to keep our beers as close to tank fresh as we possibly can.
Why four degrees? At its most basic level, this comes down to food handling standards – a lot of infrastructure around the country and indeed the world has equipment set up for this golden temperature widely regarded as “Fridge temperature”, however there is also a basis for this temperature in a scientific manner. In the book “Freshness” by Dr Charles Bamforth, he refers to the work of Swedish chemist Svant Arrhenius that relates temperature to chemical reaction speed – for beer this has been presented as a 3x faster reaction time for every ten degrees temperature increase.
What does this mean then? Down to brass tacks, for every increment of temperature increase, the chemical reactions of the live product in the container multiply rapidly. A fridge slows the microbiological and physical changes in food, and does a very similar, if not exactly the same process in beer too.
An at-home experiment you can do yourself to prove this theory is to take two cans of exactly the same beer, leave one in the fridge for a month, and one on a windowsill for a month. The fridge-stored beer will be fresher tasting, and will express the hops in a much more buoyant manner, whilst the shelf-stored beer will be noticeably dulled, bordering on undrinkable if left at the temperatures we’re currently experiencing here in the UK at the moment. A handy graph detailing this process can be found below:
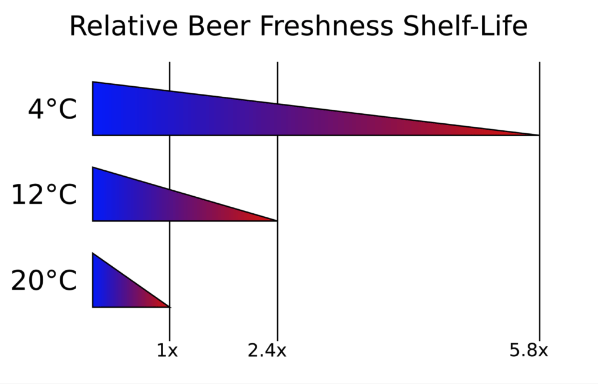
To explain what is going on in the container, we have to look at the liquid itself. Beer as previously mentioned is a very much live product; fragile to even the slightest fluctuations throughout its lifespan, and we all have a duty of care if we’re to enjoy our beer as the brewer themselves intends it to taste.
Because our beers are so highly hopped, we take great pride in the intensity of flavour that comes with the experience, however hops are also extremely sensitive to heat exposure; and ambient storage causes all those wonderful aromatics and flavour compounds to degrade rapidly in a shorter period of time when not properly stored.
It’s not only dulled-tasting beer that can rear its head with improper storage. Much more sinister effects can rear their head and create an experience that is very much the polar opposite of what we expect here at Polly’s; including but not limited to oxidation, and in very extreme cases bursting cans.
Whilst we take great care to limit the dissolved oxygen content of our beers to the lowest possible amount of parts per billion, there will always be a trace amount of oxygen in the beer and present in the headspace of a can, which our old foe temperature helps to push along any nefarious traits that may present itself in a beer.
Think of taking a bite out of an apple – initially the inside is a wonderful golden colour, but leave it exposed to oxygen for a period of time and very quickly you’ll have a brown, nasty tasting apple. This chemical reaction, known as oxidation, can happen in any beer if the elements are there to set the wheels in motion, but this becomes a much more rapidly occurring process in ambiently stored cans. An oxidised beer typically presents as brown, bordering on grey, and with a taste that can only be described as musty, wet cardboard, and in extreme cases, rotted fruits. Appealing, right?
Excessive temperature can also push along a process known as CO2 displacement through a process known as hop creep; causing the most frightening of issues we tend to see around this time of year – a bulging, or even worse, a bursting can. With average temperatures in the UK creeping up every summer, the liquid in an ambiently stored can in a garage or on a windowsill can, and often does, reach upwards of fourty degrees and above at the peak of the daytime. In the same way that rising global temperatures naturally push CO2 out of seawater, the same chemical reaction happens in a non-cold stored can of beer. The key difference however is that whereas dissolved CO2 in seawater has an atmosphere to escape into, beer only has the volumetric space of the container it’s being stored in itself.
What is hop creep then?
A process that has been noted for over a hundred years, but one that has only reared its head recently due to the influx of heavily-hopped beers that have appeared over the last ten to fifteen years, hop creep occurs when beer attenuation continues due to a dry-hop addition. Residual dextrins used to accentuate body and mouthfeel to a beer survive the brewing process and remain in situ in the finished product. Hops naturally contain enzymes that break these dextrins down into fermentable sugars, and even the smallest amount of yeast, combined with fermentation temperatures can cause a refermentation in-can.
This can not only cause the beer to become drier, affecting the intended mouthfeel, but also causes problems with additional alcohol being created thanks to the fermentation process; putting the beer wildly out of spec to the advertised ABV, and yes, causing CO2 displacement as a by product. Over time this causes pressure to build up within the can and cause a bursting can.
A follow up to the at-home experiment you can do with the fridge vs ambient test to qualify this theory is to give the cans themselves a little squeeze prior to opening. The fridge stored beer should still have some malleability to it, whilst the ambiently stored can will be almost solid to the touch – this is where a beer, specifically an unpasteurised one, can start to become unstable; and whilst aluminium cans are extremely strong – specifically around the seaming point of the can, they can only hold so much pressure before a burst happens. This process can be frightening, as they’re not usually very quiet when they do go off, and can cause damage to anything that happens to be around the can due to the pressure behind it.
So now we know the science behind it, what can we do to maintain the quality of the beer we’re drinking? We have a strong preference for little and often when it comes to beer to maintain freshness, and ensure nothing is lingering around too long. We take great pride in our cold storage set up here at Polly’s to replicate as close to what our brewers intend when they take a sample from our taps, and with proper storage you too can enjoy beer as close to how it tastes directly from tank at home too.




